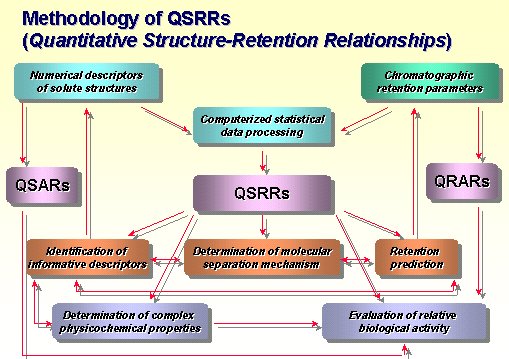
QSRRs (Quantitative Structure-Retention Relationships)
The retention mechanism of chromatographic systems depends mainly on interactions between the solutes, stationary phase and mobile phase. These interactions are explained by molecular descriptors using Quantitative Structure-Retention Relationships(QSRRs) that strives to formulate a multivariate regression equation relating molecular structure to solute retention. Recently, individual applications of QSRRs were reviewed by Kaliszan(1-2). QSRRs studies using molecular modelling concentrate two essential points on the description of retention behavior. First, one is the selection of meaningful descriptors that can only explain retention behavior, because there are so many available descriptors in QSRRs studies(1-5), such as topological, geometrical, electronic, physicochemical and intermolecular descriptors. Second, the grouping of solutes is very important process to understand the retention behaviors. In order to get a reliable and precise prediction of retention in a given chromatographic system, it is need to group the structurally similar solutes(6-7). In general, retention prediction studies have been described for structurally related homologous or substituted aromatic compounds. Pharmaceutical solutes can be completely unrelated in structure and contain various functional group, which makes the retention prediction less accurate(8).
Reference
- R. Kaliszan, J. Chromatogr. A, 1993, 656, 417.
- R. Kaliszan, Anal. Chem. 1992, 64, 619A.
- R. Kaliszan, and K. Osmialowski, J.Chromatogr., 1990, 506, 3.
- H. S. Kim, T. K. Kim and D. W. Lee, J.Liq. Chromatogr., 1994, 17, 2615.
- S. K. Lee, Y. H. Park, C. J. Yoon and D.W. Lee, J. Micro. Sepn., 1998, 10(1), 133.
- Z. L. Sun, M. C. Liu and Z. D. Hu, Chromatographia, 1994, 38, 599.
- T. Hanai, H. Hatano, N. Nimura and T. Kinoshita, Analyst, 1994, 119, 1167.
- Retention and Selectivity in Liquid Chromatography, R. M. Smith, (Elsevier, Amsterdam, 1995) p47.
